Thermal-Oxidative Degradation of Polymers
Most polymers will undergo significant changes over time when exposed to heat, light, or oxygen. These changes will have a dramatic effect on the service life and properties of the polymer and can only be prevented or slowed down by the addition of UV stabilizers and antioxidants. The degradation of polymers can be induced by
- Heat (thermal degradation)
- Oxygen (oxidative and thermal-oxidative degradation)
- Light (photodegradation)
- Weathering (generally UV/ozone degradation)
The deterioation due to oxidation and heat is greatly accelerated by stress, and exposure to other reactive compounds like ozone.
All polymers will undego some degradation during service life. The result will be a steady decline in their (mechanical) properties caused by changes to the molecular weight and molecular weight distribution and composition of the polymer. Other possible changes include:
- Embrittlement (chain hardening)
- Softening (chain scission)
- Color changes
- Cracking and charring (weight loss)
In general, the resistance to degradation will depend on the
chemical composition of the polymer. For example, polymers such as
polypropylene (PP), polyvinylchloride (PVC), and polybutadiene (PBD)
are very susceptible to degradation and can only withstand UV, oxidative and thermal degradation / decomposition
when formulated with UV stabilizers and antioxidants, whereas polymers such as
polysulfone (PES, PSU), polyetherketone (PEEK), and polysiloxanes
(silicones) possess excellent resistance to thermal and oxidative
degradation due to the strong bonds in the long chain backbone and
in the side-groups.
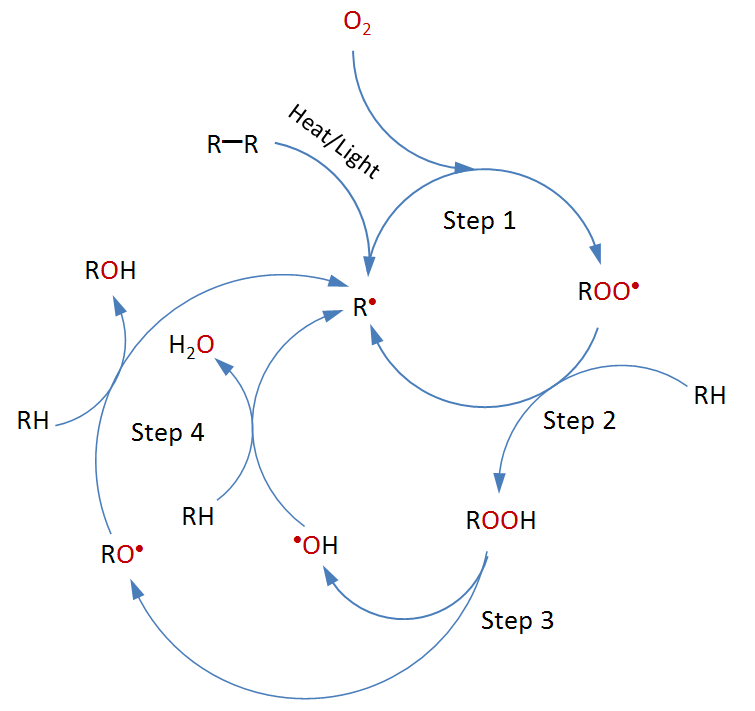
The general mechanism of thermal-oxidative degradation of
polymers is shown below.
General mechanism of thermal degradation
Initiation
Oxidative degradation is usually initiated when polymer chains form radicals, either by hydrogen abstration or by homolytic scission of a carbon-carbon bond. This can occur during manufacture, processing or during service when exposing the polymer to light or heat.
R−H → R· + H·
Propagation
The propagation of thermal degradation involves a number of reactions. The first step is the reaction of a free radical (R·) with an oxygen molecule (O2) to form a peroxy radical (ROO·*), which then abstracts a hydrogen atom from another polymer chain to form a hydroperoxide (ROOH). The hydroperoxide splits then into two new free radicals, (RO·) + (·OH), which abstract labile hydrogens from other polymer chains. Since each initiating radical can produce two new free radicals, the process can accelerate depending on how easy it is to remove hydrogen from other polymer chains and how quickly free radicals undergo termination via recombination and disproportionation.
R· + O2 → ROO·
ROO· + RH → R· + ROOH
ROOH → RO· + ·OH
RO· + RH → R· + ROH
·OH + RH → R· + H2O
Termination
Termination of thermal degradation is achieved by recombination
of two radicals or by disproportionation / hydrogen abstraction. These reactions always
occur but can be accelerated by additon of stabilizers.
Recombination of two chain radicals results in an increase of the
molecular weight and crossilinking density:
R· + R· → R−R
2 ROO· → ROOR + O2
R· + ROO· → ROOR
R· + RO· → ROR
HO· + ROO· → ROH + O2
The result of these reaction is enbrittlement and cracking of the polymer. Termination by chain scission, on the other hand, results in the decrease of the molecular weight leading to softening of the polymer and reduction of the mechanical properties
Rn· + Rm· → Rn-2−CH=CH2 + Rm
2 RCOO· → RC=O + ROH + O2
Which of these termination steps is predominant will depend on the type of polymer and on the conditions. For example, polyolefins with short alkyl side groups like polypropylene and polybutylene, and unsaturated polymers like natural rubber (polyisoprene) undergo predominantly chain scission, whereas polyethylene, and rubbers with somewhat less active double bonds like polybutadiene and polychloropene suffer from embrittlement due to crosslinking during aging.